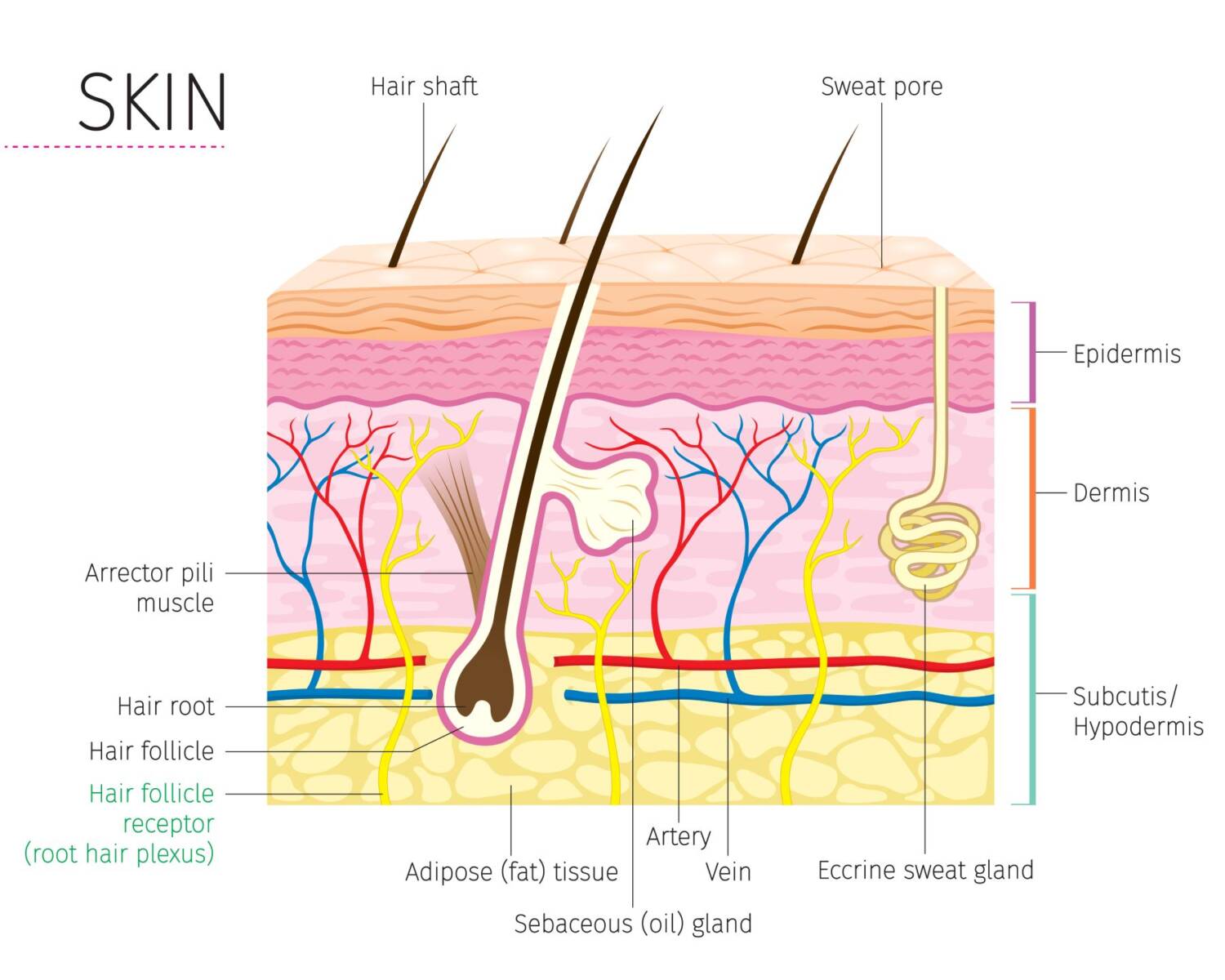
Seeing Skincare Differently
Our skin, the largest organ of our body, is much more than what we see on the surface. It’s a complex and crucial barrier that

For All Skin Types

Newly discovered understanding of the microbiome and its relationship to our health and wellness opens the door to a new approach in treating and curing diseases of the skin and more. Our “Under The Microscope” series is meant to engage our colleagues in the conversation for change in the paradigm of how we define health and wellness initiatives. Are we ready to begin?
Much of the time and money being spent on creating new drugs, cosmeceuticals, devices and other new products is wasted due to the obsolescence of testing products on animals.
Our governing agencies along with big pharma’s domination of them, have become obstacles to product development, and thus are now a hindrance to progress. I believe the time has come to totally rethink the system of product testing on animals.
Animal testing has been an integral phase of pre-clinical trials for every major drug on the market today. In fact, the FDA requires that new drugs go through various stages of animal testing before they can move on to human trials. These protocols were created long ago, when we did not have the knowledge and understanding which we have today. Furthermore, the ethical concerns related to animal testing along with the biological differences between trial subjects make these tests a most inappropriate way of modeling the effects of drugs in humans.
Here is an illustration of our present system at work. A team of researchers at a global pharmaceutical company were deep into pre-clinical research of a new drug when an event occurred which resulted in the end of the project. The novel molecule in development had passed rodent testing and was now being trialed on dogs. The dogs developed a canine jaw snapping side effect which spelled doom for this project, as the researchers could not figure out why this was occurring in the dogs and thus they could not explain to the FDA why this drug merited further trials.
This illustrates what can go wrong when you use animal studies to predict safety studies for products designed for humans. Alternative methods could have and should have been engaged in the evaluation of this new drug. The science is now readily available which can provide better evaluation of the safety and efficacy of a new drug for human use.
This entire process of testing on animals is both outdated and rarely if ever predictive in humans while also being cruel to animals. Furthermore, nearly 90% of drugs in human trials fail despite showing positive responses in animal tests! Why do we continue testing new products on animals anyway? There are a number of explanations for this, and none of them are justifiable based on our current knowledge.
Today we have many new technologies for improving drug development testing including computational models, “organ on a chip,” advanced cell culture assays, and more. Each of these methodologies represent an advancement in the evaluation process and they would likely reduce the costs and increase the efficiency of new product development.

Time has come today to begin the transformation of the drug discovery process and to begin incorporating new modalities into the clinical trial processes. Animal testing is not and has not been the best method for product testing. Having a human relevant model will increase the probability of success for new products while also saving the animals from suffering. That is what should matter at the end of the day.

Our skin, the largest organ of our body, is much more than what we see on the surface. It’s a complex and crucial barrier that

In the world of skincare, countless products vie for our attention, promising a youthful glow, wrinkle reduction, and age defying perfection. But with so many

In the realm of skincare shopping, there’s a little-known secret that can make a big difference both to your wallet and the planet: opting for

The “wellness” industry is booming. From colon cleanses to crystal healing, the market explodes with promises of optimal health, inner peace, and eternal youth. But

At Columbia SkinCare, when we ponder the question, “Are you feeling well?”, we delve deep into the essence of what wellness truly signifies. In our